Covid-19 Pandemic
  Here’s the latest news: Signs Britain may end 2-meter rule and loosen lockdownAstraZeneca reaches vaccine supply deal with four countriesBeijing locks down part of city after virus outbreak at market A conversation with J&J’s vaccine hunter Despite decades studying diseases like HIV, Ebola and tuberculosis, Dr. Paul Stoffels says the breakneck speed at which Covid-19 spread around the world was “completely unexpected.” Stoffels, the chief scientific officer of drug giant Johnson & Johnson, says he’s now putting his long experience researching infectious diseases and developing drugs and vaccines to work for one ambitious goal: “Putting an end to this epidemic.” The Belgian doctor-turned-executive is leading the development of an experimental vaccine that’s received a $456 million award from BARDA, the U.S. agency responsible for developing medical countermeasures to emergencies. The White House recently tapped J&J as one of a select few it will provide additional resources and aid to get a shot across the finish line. The drug giant has suggested it is closer than previously anticipated to that goal. Here’s the latest news: Signs Britain may end 2-meter rule and loosen lockdownAstraZeneca reaches vaccine supply deal with four countriesBeijing locks down part of city after virus outbreak at market A conversation with J&J’s vaccine hunter Despite decades studying diseases like HIV, Ebola and tuberculosis, Dr. Paul Stoffels says the breakneck speed at which Covid-19 spread around the world was “completely unexpected.” Stoffels, the chief scientific officer of drug giant Johnson & Johnson, says he’s now putting his long experience researching infectious diseases and developing drugs and vaccines to work for one ambitious goal: “Putting an end to this epidemic.” The Belgian doctor-turned-executive is leading the development of an experimental vaccine that’s received a $456 million award from BARDA, the U.S. agency responsible for developing medical countermeasures to emergencies. The White House recently tapped J&J as one of a select few it will provide additional resources and aid to get a shot across the finish line. The drug giant has suggested it is closer than previously anticipated to that goal.   Paul Stoffels, chief scientific officer of Johnson & Johnson, in 2017. Photographer: Michele Limina/Bloomberg Stoffels, 58, began his career as a physician in Africa focusing on HIV and tropical diseases. After joining J&J in 2002, he spearheaded development of several HIV drugs and worked on inoculations for Ebola and Zika. He spoke to Bloomberg News about the race for a Covid-19 vaccine. What is the minimum rate of efficacy you believe should be reached for a coronavirus vaccine to be considered useful, or successful? Paul Stoffels, chief scientific officer of Johnson & Johnson, in 2017. Photographer: Michele Limina/Bloomberg Stoffels, 58, began his career as a physician in Africa focusing on HIV and tropical diseases. After joining J&J in 2002, he spearheaded development of several HIV drugs and worked on inoculations for Ebola and Zika. He spoke to Bloomberg News about the race for a Covid-19 vaccine. What is the minimum rate of efficacy you believe should be reached for a coronavirus vaccine to be considered useful, or successful?STOFFELS: There’s a debate which is ongoing in the medical community: Is 50% enough? Does it have to be 70%? Does it have to be 90%? If you can prevent 7 out of 10 people from getting infected or getting sick, it’s valuable to do a large vaccination, and that’s not up to us to say that, but that is what the assumption of the authorities is today that we go for a 70% efficacy. It’s a common, accepted number at the moment for a target. J&J has signed on collaborators to build out manufacturing capacity so that it can produce one billion doses before the end of 2021. Do you have enough capacity already to reach that objective? STOFFELS: With our current capacity, we are almost there, but we want to go further to have more capacity if need be for the world. So we are at least going do two additional collaborations with either CMOs, contract manufacturing organizations, or with partners to build additional capacity for getting up to significantly above a billion vaccines a year. Now for vaccine manufacturing, you have two parts: You have what is called vaccine substance, which is the vaccine itself, and then you actually have to fill it. And that process from vaccine substance to filling to release is several months. That process starts later in the year, toward an October or November time-frame, when we will actually start manufacturing large scale batches of vaccines. J&J has received significant funding from the U.S. government. Have you made any formal commitments to the country should the vaccine prove successful? STOFFELS: There is no contract that says the U.S. will be first, but we needed to expand very significant the manufacturing capacity. There was a site available in the U.S. already funded by BARDA, in the past. We contracted that site, and that does accelerate the availability of vaccines the U.S. J&J has committed to offer its vaccine on a not-for-profit basis amid the pandemic. What kind of at-risk investments and manufacturing costs will the company include in the ultimate price-tag, and would you agree to disclose those costs? STOFFELS: We are working on that and at a certain point we’ll disclose and make that public. We are not yet ready for that as we are still working on our cost structure in the world, on what it will take to get it all done. But we will be public about how we do this. We commit to equitable access globally, as well as to volume to make sure people can get access on a global scale.–Riley Griffin Track the virus Tracking the U.S. Small Business Virus Impact Small businesses, the beating heart of the U.S. economy, have been hard hit by the fallout from the coronavirus pandemic. About $349 billion in federal relief loans designed to help them survive amid shutdowns ran out in just 13 days in April, with many mom-and-pop stores being shut out. 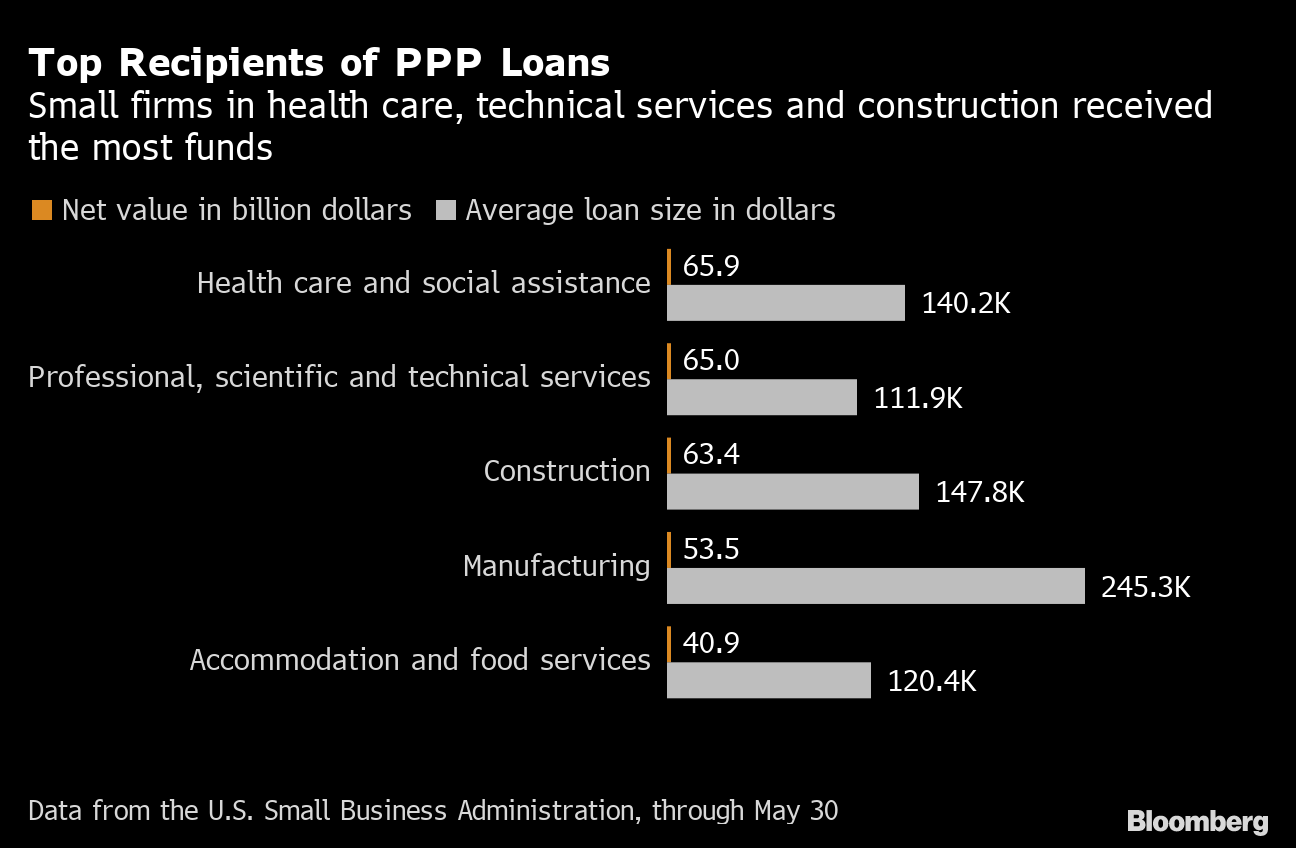  Coming events: Can the world’s medical researchers, scientists and public policy advocates work in tandem to outwit the virus and health challenges that now threaten mankind? Join us on June 16 at 10 a.m. EDT as we discuss how to overcome these crises. See the full lineup and register here. On June 18th, hear from senior leaders including Dame Jayne-Anne Gadhia of Snoop and Johann Butting of Slack on how they bolstered digital offerings and positioned for the pandemic amid a surging need for digital technologies. Get details and register here. What you should read Surge in Cases Can’t Keep Miami Beaches Empty Most people including the Florida governor aren’t wearing masks. Lessons on Virus From Asia’s Most Dense Slum Authorities knock on 47,500 doors near Mumbai in India to gauge health. After Beating Virus, Greece Seeks Tourists Nation looking to save the summer season and its economy. Businesses Transformed by Virus Keep Changes Plumbers and cleaners are thriving in the Covid-19 downturn. Virus Cases are Jumping Around the Globe From China to Russia and the U.S., infection rates are rising in places. Know someone else who would like this newsletter? Have them sign up here. Have any questions, concerns, or news tips on Covid-19 news? Get in touch or help us cover the story. Like this newsletter? Subscribe for unlimited access to trusted, data-based journalism in 120 countries around the world and gain expert analysis from exclusive daily newsletters, The Bloomberg Open and The Bloomberg Close. Follow Us Get the newsletter You received this message because you are subscribed to Bloomberg’s Coronavirus Daily newsletter. Unsubscribe | Bloomberg.com | Contact Us Bloomberg L.P. 731 Lexington, New York, NY, 10022 Coming events: Can the world’s medical researchers, scientists and public policy advocates work in tandem to outwit the virus and health challenges that now threaten mankind? Join us on June 16 at 10 a.m. EDT as we discuss how to overcome these crises. See the full lineup and register here. On June 18th, hear from senior leaders including Dame Jayne-Anne Gadhia of Snoop and Johann Butting of Slack on how they bolstered digital offerings and positioned for the pandemic amid a surging need for digital technologies. Get details and register here. What you should read Surge in Cases Can’t Keep Miami Beaches Empty Most people including the Florida governor aren’t wearing masks. Lessons on Virus From Asia’s Most Dense Slum Authorities knock on 47,500 doors near Mumbai in India to gauge health. After Beating Virus, Greece Seeks Tourists Nation looking to save the summer season and its economy. Businesses Transformed by Virus Keep Changes Plumbers and cleaners are thriving in the Covid-19 downturn. Virus Cases are Jumping Around the Globe From China to Russia and the U.S., infection rates are rising in places. Know someone else who would like this newsletter? Have them sign up here. Have any questions, concerns, or news tips on Covid-19 news? Get in touch or help us cover the story. Like this newsletter? Subscribe for unlimited access to trusted, data-based journalism in 120 countries around the world and gain expert analysis from exclusive daily newsletters, The Bloomberg Open and The Bloomberg Close. Follow Us Get the newsletter You received this message because you are subscribed to Bloomberg’s Coronavirus Daily newsletter. Unsubscribe | Bloomberg.com | Contact Us Bloomberg L.P. 731 Lexington, New York, NY, 10022 |
PreviousCovid-19 Pandemic