Covid-19 Pandemic
  Here’s the latest news: Chinese city near North Korea partly shut on virus spreadElon Musk sues California county over shutdown Obama blasts Trump’s virus response as ‘chaotic disaster’ Our take on the latest developments U.S. regulators had to reverse themselves last week after moves to solve mask shortages and speed antibody tests to market backfired—a sign that even in a pandemic, it can pay for health officials to take their time. The Food and Drug Administration has been criticized in the past for being deliberate, and it was blamed for slowing the release of diagnostic tests in the early stages of the pandemic with needless bureaucracy. When Covid-19 began to spread in the U.S., it looked to remove some roadblocks. Now, the agency is undoing a mid-March policy that allowed antibody tests, which can tell whether someone has ever been infected with the new virus, to be sold without FDA review. The change opened the door for fraudulent tests that didn’t work like they claimed. Here’s the latest news: Chinese city near North Korea partly shut on virus spreadElon Musk sues California county over shutdown Obama blasts Trump’s virus response as ‘chaotic disaster’ Our take on the latest developments U.S. regulators had to reverse themselves last week after moves to solve mask shortages and speed antibody tests to market backfired—a sign that even in a pandemic, it can pay for health officials to take their time. The Food and Drug Administration has been criticized in the past for being deliberate, and it was blamed for slowing the release of diagnostic tests in the early stages of the pandemic with needless bureaucracy. When Covid-19 began to spread in the U.S., it looked to remove some roadblocks. Now, the agency is undoing a mid-March policy that allowed antibody tests, which can tell whether someone has ever been infected with the new virus, to be sold without FDA review. The change opened the door for fraudulent tests that didn’t work like they claimed.   Stephen Hahn, commissioner at the Food and Drug Administration since December. Photographer: Andrew Harrer/Bloomberg Similarly, the agency found N95 masks made in China that don’t provide proper protection. As a result, it reversed a policy change that allowed them on the market based on testing from third-party labs. Stephen Hahn, commissioner at the Food and Drug Administration since December. Photographer: Andrew Harrer/Bloomberg Similarly, the agency found N95 masks made in China that don’t provide proper protection. As a result, it reversed a policy change that allowed them on the market based on testing from third-party labs.The conflict over whether FDA regulations hinder or help is an old one, but it’s been heightened during the Trump administration. For example, the president himself pushed Right to Try legislation backed by the libertarian Goldwater Institute in an effort to expand access to experimental drugs and forgo normal FDA protocols. Vaping also fits in this category. Former FDA Commissioner Scott Gottlieb didn’t want the agency to potentially hinder a new technology, so pushed back FDA reviews of the products by several years. In the meantime, the U.S. experienced an epidemic of youth vaping. More reversals could be in store. The agency issued an emergency authorization for the malaria drugs hydroxychloroquine and chloroquine to be used against Covid-19, lacking evidence they work. Clinical-trial results due soon could prompt more action.—Anna Edney Report card Most States Fall Short of Reopening Criteria Around the U.S., states are debating whether and how to reopen their economies and lift social-distancing measures. But are they ready? See how states are faring by a number of key metrics. 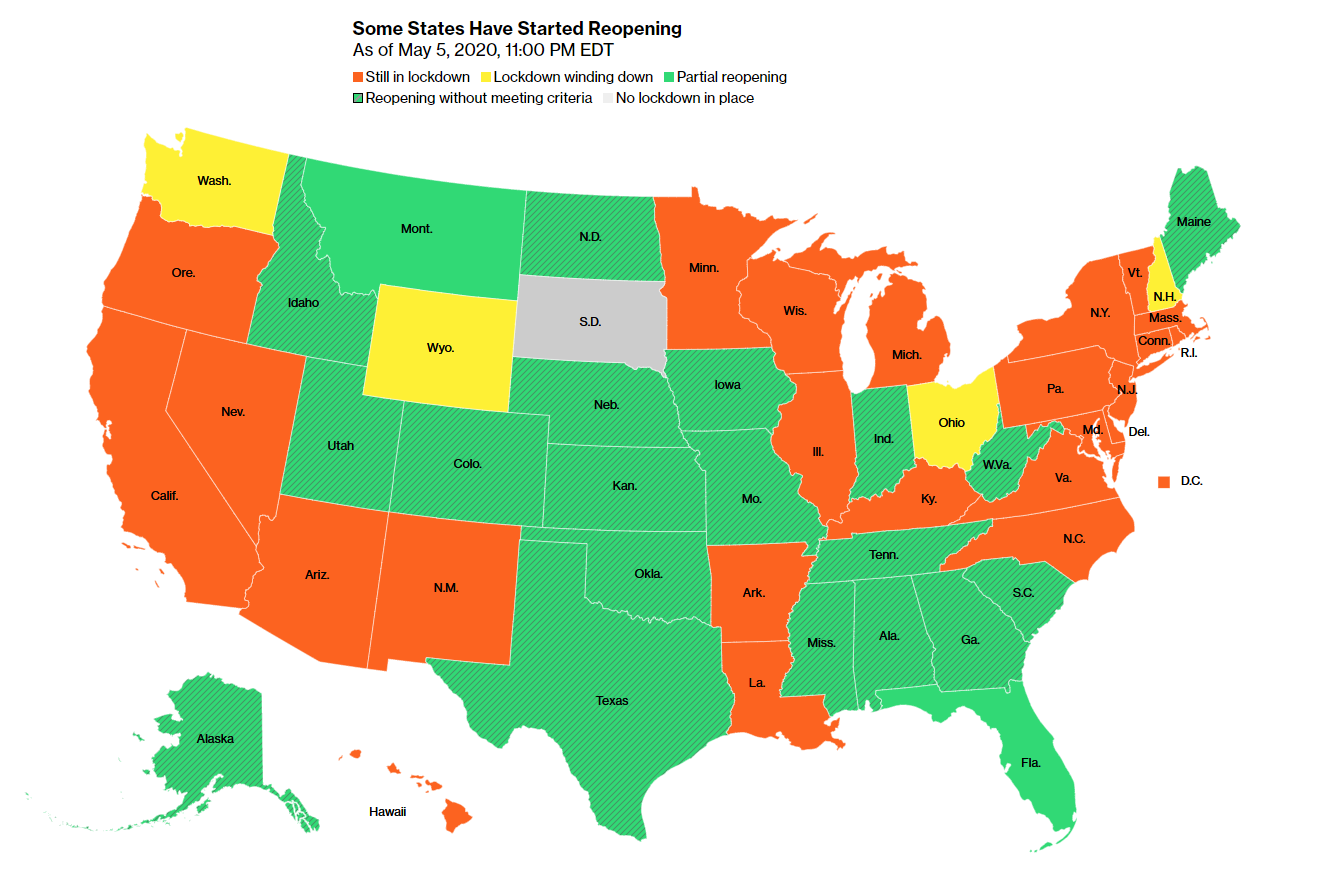  Bloomberg Bloomberg What you should read Europe’s Economy Faces Long Slog Tentative easing looks likely to herald slow recovery, not snapback. U.S. Airlines Endorse Fever Checks Controls could make it harder to assure customers that it’s safe to fly. Boris Johnson to Set Out Virus Warning System New measures will force those arriving in U.K. to self-isolate for 14 days. Stock Markets Not Likely to Indicate End of Crisis Earnings forecast still too optimistic and market volatility likely, RBC says. Hidden Defaults to Soar as Companies Squeezed Companies may default in ways that fly under the general public’s radar. Know someone else who would like this newsletter? Have them sign up here. Have any questions, concerns, or news tips on Covid-19 news? Get in touch or help us cover the story. Like this newsletter? Subscribe for unlimited access to trusted, data-based journalism in 120 countries around the world and gain expert analysis from exclusive daily newsletters, The Bloomberg Open and The Bloomberg Close. Follow Us Get the newsletter You received this message because you are subscribed to Bloomberg’s Coronavirus Daily newsletter. Unsubscribe | Bloomberg.com | Contact Us Bloomberg L.P. 731 Lexington, New York, NY, 10022 Bloomberg Bloomberg What you should read Europe’s Economy Faces Long Slog Tentative easing looks likely to herald slow recovery, not snapback. U.S. Airlines Endorse Fever Checks Controls could make it harder to assure customers that it’s safe to fly. Boris Johnson to Set Out Virus Warning System New measures will force those arriving in U.K. to self-isolate for 14 days. Stock Markets Not Likely to Indicate End of Crisis Earnings forecast still too optimistic and market volatility likely, RBC says. Hidden Defaults to Soar as Companies Squeezed Companies may default in ways that fly under the general public’s radar. Know someone else who would like this newsletter? Have them sign up here. Have any questions, concerns, or news tips on Covid-19 news? Get in touch or help us cover the story. Like this newsletter? Subscribe for unlimited access to trusted, data-based journalism in 120 countries around the world and gain expert analysis from exclusive daily newsletters, The Bloomberg Open and The Bloomberg Close. Follow Us Get the newsletter You received this message because you are subscribed to Bloomberg’s Coronavirus Daily newsletter. Unsubscribe | Bloomberg.com | Contact Us Bloomberg L.P. 731 Lexington, New York, NY, 10022 |
PreviousCovif-19 Pandemic